What Is A Biomarker?
Defining Biomarkers
The word biomarker is short for biological marker. A biomarker is any entity (thing) in the body that can take a snapshot or measure what is happening in the body at a given moment. Biomarkers can serve as early warning systems for your health.
For example, high cholesterol levels are a common biomarker for heart disease risk, and high levels of lead in the bloodstream show a possible need to test for nervous system and cognitive disorders, especially in children.
Biomarkers play an important role in helping us understand relationships between environmental exposures, human biology, and disease. Biomarkers can help us diagnose disease, choose therapy, and follow patients for treatment response, disease recurrence, and outcome. Scientists can use biomarkers to turn research findings into something useful in medicine and public health.
Biomarker Testing
Doctors often look at how changes in genes or proteins in a person’s cancer might affect their treatment options. We call this biomarker testing, where genes or proteins are the biomarkers.
Of note: Genes are pieces of DNA inside a cell. Each gene contains a code (instructions) needed to make a certain protein. Each protein has a given job to do in that cell.
(The word genes sounds like “jeens” when we say it. The word “proteins” sounds like “prohteens” when we say it.)
In biomarker testing, a sample of a patient tumor (cancer) is usually taken. Sometimes testing is done in blood. The use of biomarkers in the blood is being studied by many scientists and is growing quite quickly; however, currently, its use for treatment choice is limited. Biomarker testing in the blood is sometimes used to follow response to therapy and/or detect recurrence.
Biomarkers in tumor tissue can guide treatment choice and sometimes predict how well a patient might respond to treatment.
Value Of Testing And Who Should Be Tested
Finding out what biomarkers are in a tumor is critical for any patient because actionable (usable) biomarkers inform a patient’s treatment team whether additional therapies besides chemotherapy will might improve the outcome for that individual patient. These additional therapies may include immunotherapy, targeted therapy, or others. In some cases, these “tailored” treatments may give better results than the general chemotherapy that is offered to all patients regardless of biomarkers.
Biomarker tests can be quite helpful to people after they have been diagnosed with stomach cancer (See STOMACH CANCER: BIOMARKER-GUIDED TREATMENTS chart). If anyone with stomach cancer, particularly newly diagnosed disease, is unsure about any part of biomarker testing, they should discuss it with their doctor.
Stomach cancer biomarker testing offers the following value:
- It helps decide the best initial treatment. If a patient’s cancer contains one of the established biomarkers, medicines specially targeted to these biomarkers might be the best treatment to select. In some cases, response to these targeted treatments may be better than with general chemotherapy, which is not “personalized” in this way.
- It helps avoid ineffective treatments. Biomarker results may also indicate that a person’s cancer does not have actionable (usable) biomarkers, meaning some treatments are unlikely to work. This information can help avoid treatments that will not be effective for that person’s specific tumor.
- It helps predict the risk of the tumor. Biomarker test results might suggest how a person is likely to benefit from treatment in general without pinpointing a particular therapy. For example, certain biomarkers might point out that a person’s cancer is aggressive and likely to return after initial treatment. In such cases, a patient and provider would be more empowered to discuss additional or more aggressive treatment options.
- It helps to can more quickly detect when a tumor comes back. After anticancer treatment has ended, and the cancer seems to have gone, blood samples can be collected from time to time and tested for tumor markers to check for recurrence (the return of cancer).
- It helps to identify next steps when standard treatments don’t work. If a patient’s stomach tumor does not shrink or it comes back after initially responding to treatment and is no longer sensitive to stomach cancer-approved therapies, biomarkers may identify medicines approved for other diseases that could be used “off-label”. In this setting, biomarkers can may also identify clinical trials of new medicines that patients may might be eligible for and benefit from.
For all these reasons, biomarker testing can be quite helpful at every phase of the journey taken by a person with stomach cancer. If anyone with stomach cancer, particularly if newly diagnosed, is unsure about any part of biomarker testing, they should discuss it with their doctor.
Why And Who Should Test
If a patient’s cancer contains one of the established biomarkers, treatments targeted to these biomarkers can be prescribed (personalized or “made-to-order” therapy). Patient response to these targeted treatments may be better than with general chemotherapy that is not “made-to-order.”
Biomarker tests can be quite helpful to people after they have been diagnosed with stomach cancer. If anyone with stomach cancer, particularly newly diagnosed, is unsure about any part of biomarker testing, they should discuss it with their doctor.
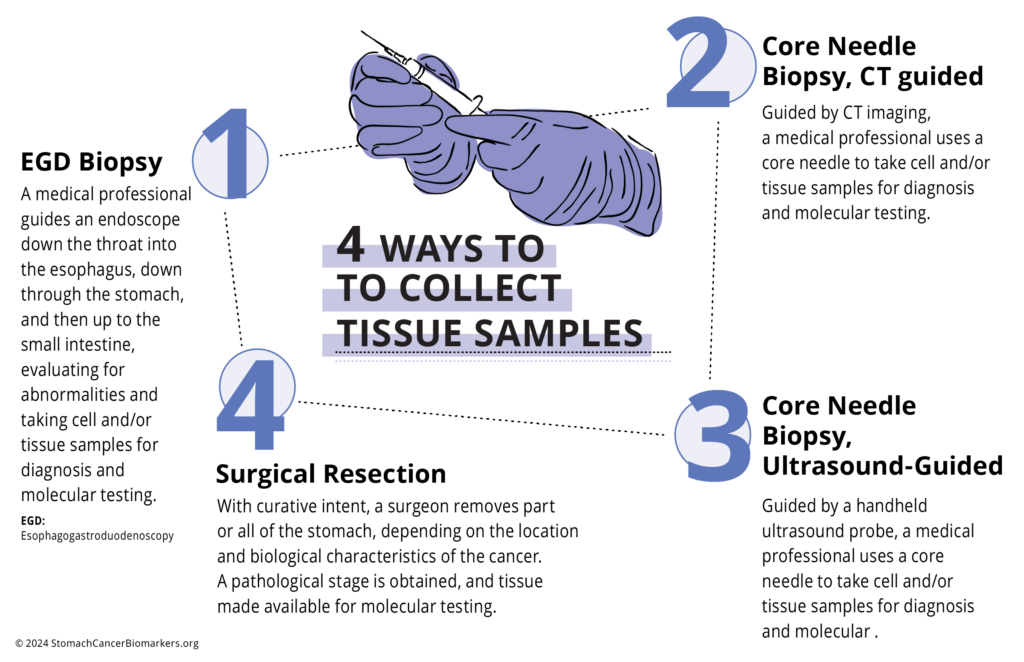
What To Expect When Testing
Biomarker testing for treatment choice might not be so available in some places.
Even when biomarker testing is available to look for gene or protein changes that might help treatment choice, a person might not have their tumor tested for these changes for a number of reasons.
It is important for the person with stomach cancer (the patient) and their loved ones to ask questions and know all their options. They should ask their healthcare team the following questions about the patient:
- Should I have cancer biomarker testing done?
- What information will the tests help you find out?
- Will the tests help us choose which treatments are best for me?
- How much will the tests cost? Will my insurance cover them?
- If testing shows a specific treatment might be best for me, how much will this treatment cost? Will my insurance cover it?
In Tumor Tissue
Ways of measuring HER2
HER2 protein amounts are measured using IHC.
HER2 gene amounts “amplification” are measured using NGS or ISH.
Ways of measuring PD-L1
PD-L1 protein levels are tested using IHC.
Ways of measuring MSI-H/dMMR
MSI is tested by PCR or NGS.
MMR mutations are tested by IHC.
Ways of measuring TMB
High tumor mutational burden (TMB) is tested by NGS.
Explanation Of Technical Terms
IHC = immunohistochemistry; ISH = in situ hybridization; NGS = next-generation sequencing; PCR = polymerase chain reaction.
IHC is a laboratory method that uses antibodies to check for certain antigens (biomarkers such as HER2) in a tumor sample.
In our bodies, an antibody is an immune system protein that responds and binds to another protein called an antigen, causing an immune response. Each antibody can bind to only one specific antigen.
IHC involves using a similar antibody-antigen process. In IHC, an antibody locates antigens (e.g., HER2) in cancer biopsy tissue through the use of a visual marker. Fluorescent dye is the most common visual marker used, although there are others.
PCR is a laboratory method that makes millions of copies of a DNA sample so that scientists can take a very small sample and make it big enough to see in detail.
ISH is used in the laboratory to detect specific DNA (and RNA) sequences in cells. In the case of cancer biomarkers, in situ hybridization detects a specific mutated DNA sequence and the number of copies of this mutated DNA.
NGS is used in high-tech laboratories to detect specific DNA (and RNA) sequences in cells. In the case of cancer biomarkers, NGS detects a specific mutated DNA sequence and the number of copies of this mutated DNA. This technology can be used on a large scale.
*It is important that all tests are done in CLIA-certified laboratories.
*CLIA = Clinical Laboratory Improvement Amendments.
Only CLIA-certified labs meet federal regulations for clinical diagnostic testing, ensuring the quality of results.
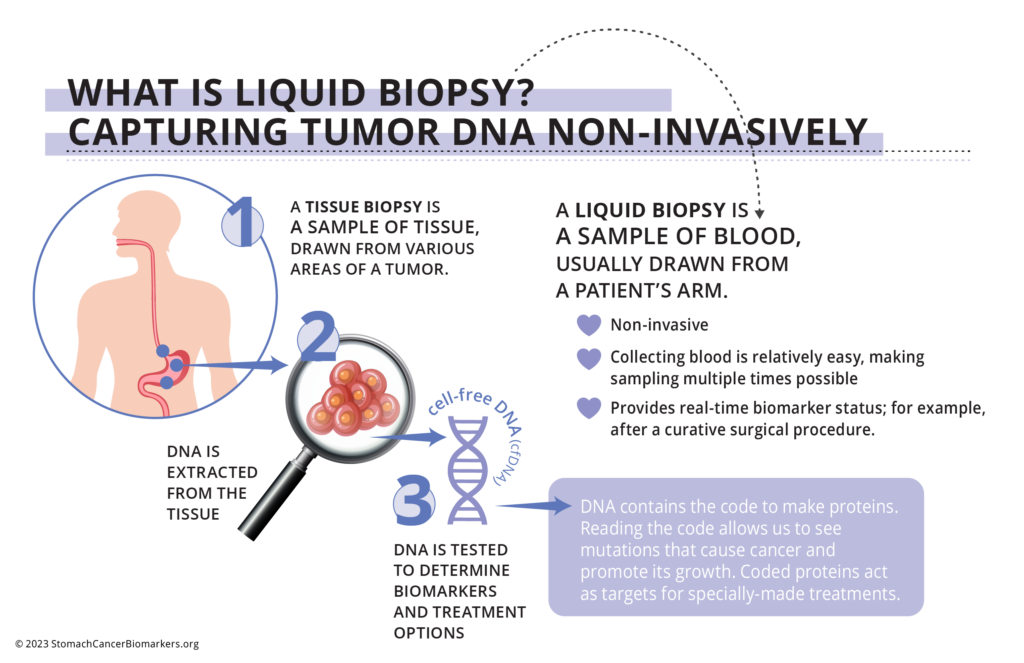
Liquid Biopsy
Liquid biopsy—circulating tumor DNA (ctDNA) in a blood sample.
Liquid biopsy is now being used in patients with advanced gastric/GEJ cancer. The testing of biomarkers in blood can be done using NGS.
Biomarker testing in the blood can be complementary to tumor tissue-based testing, as both approaches can have limitations that the other can compensate for.
Results: What Testing Shows
Testing of all patients for HER2, PD-L1, and MSI biomarkers should be done when a patient is first diagnosed and if metastatic disease is confirmed or suspected. PD-L1 testing should be done in patients with recurrent or mGC (see “For patients with stage 4 (metastatic) and some stage 3 (locally advanced unresectable or recurrent) gastric/GEJ cancers” below).
For patients with stages 0, 1, 2, or some stage 3 gastric/GEJ cancers:
Removal of the tumor using surgery is the most basic route to a possible cure.
– Depending on the specific cancer diagnosis, location, and stage, additional therapies like radiation therapy or systemic therapy (chemotherapy, targeted therapy, immunotherapy) may might help shrink the tumor and make it easier for the surgeon to carry out successful curative surgery. These therapies are often given before but can sometimes be given after the surgery.
For example:
- One or a combination of systemic therapies is used for stage 2 or 3 cancer.
- Systemic therapy might also be used for stage 1 cancer.
- There are open clinical trials of immunotherapy and biomarker-targeted treatment if a patient is “a fit” for any of these trials (you will hear doctors patients will often hear doctors talking about being “eligible” or “meeting the criteria”) and if the treating physician thinks the trial is a good idea for that particular patient.
Stage 0 cancer is abnormal cells that look like cancer cells under a microscope. These cells are found only in the stomach and haven’t spread to nearby tissue.
Stage 1 cancer is generally small, contained within the stomach, and has not spread to the lymph nodes or other parts of the body).
Stage 2 or 3 cancers are both classed as locally advanced and have some degree of spread into other tissues but can be surgically removable.
For patients with stage 4 (metastatic) and some stage 3 (locally advanced unresectable or recurrent) gastric/GEJ cancers:
- Surgery typically has more risks than benefits and can only take out one area of the tumor. Unfortunately, if a cancer has spread to multiple sites, it is rare that a surgeon can safely and successfully remove all cancer cells within a patient. For this reason, we must instead rely on therapies that can access the entire body, which is referred to as systemic therapy. This therapy is discussed in the next section (locally advanced, recurrent, or metastatic cancer treatments)
Metastatic means that the cancer has spread to other parts of the body
Locally advanced unresectable means that the cancer has spread within the stomach/GEJ in a way that cannot be removed by surgery
Recurrent means the cancer has returned after previous therapy.
Risks Of Testing
It may not be possible to get results. Sometimes, a person may not be able to have a biopsy taken safely or have a biopsy taken that does not contain enough tumor tissue to do biomarker testing. Additionally, depending on where a patient lives and gets treated, certain biomarker tests may not be readily available.
- The results may not be helpful. Although biomarker testing can sometimes help find the best cancer treatment for the person being tested, this testing does not always help everyone. There are various reasons for this, including:
- Sometimes, the biomarkers in a tumor do not match any available therapies.
- Sometimes, the biomarkers in a tumor reflect changes that are not useful in making treatment decisions because their significance in cancer is not clearly understood (known as variants of unknown significance, or VUS).
Sometimes, a matching therapy may be found, but it is off-label (it is FDA-approved to treat a different disease) and not covered by a person’s health insurance.
- Sometimes, the matching therapy is a new therapy that is still in clinical trials, and the person may not be eligible or able to participate.
- Even if a biomarker matches an available treatment, there is no guarantee that the therapy will be effective for that person. Many factors can influence how successful a treatment is, such as how the cancer behaves and how a person’s body responds to the treatment.
- The results may change. Biomarker testing of tumor tissue provides a single “snapshot” of the biomarkers present at the time of biopsy. The biomarkers can then change. So, what we see today may be different from what we see further down the line. If a cancer reappears after treatment, a healthcare provider may recommend doing another biomarker test to see the latest picture and change therapy as needed.
Cost Of Testing
The cost of biomarker testing and the treatment choices made as a result of this testing can sometimes be a concern:
- Tests for gene and protein changes can be expensive, especially if many changes are being assessed, and insurance might not cover all testing costs.
- Moreover, if a person’s or people whose best treatment option based on their cancer’s gene or protein changes is a specific targeted therapy or immunotherapy based on their cancer’s gene or protein changes, the treatment itself might be expensive and might not be covered by insurance.
More information* on insurance coverage:
- For people with advanced cancer, some biomarker tests are covered by Medicare and Medicaid.
- Private insurance companies often cover the cost of biomarker testing if there is enough proof that the test is needed to guide treatment decisions. See the download of approved biomarkers.
- Tests without enough proof to support their value may be viewed as experimental. In these cases, insurance might not cover the tests or the biomarker-suggested treatment. This type of biomarker testing might be done as part of a clinical trial of a new cancer treatment.
- If a person joins one of these clinical trials, the cost of biomarker testing for any new biomarkers and the recommended treatments should be covered by the study.
*A person’s healthcare team can provide more specific information about related costs and what a person’s insurance will cover. They can also provide clinical trial information

